Stable Isotope Geochemistry
Stable isotopes are atoms of the same element with different neutron counts (e.g., 12C, 13C). The 13C/12C ratio is expressed in δ‑notation relative to an international standard and shifts during physical and biological processes. We use isotopes to trace carbon and water cycling, partition greenhouse‑gas sources, and reconstruct past climates.
We measure isotope ratios with isotope‑ratio mass spectrometry (IRMS) and laser‑based spectroscopy. IRMS ionises the sample, separates isotopologues in a magnetic field and detects them simultaneously with multiple Faraday cups. Optical instruments such as cavity ring‑down probe isotopologues directly (e.g., 12CH4, 13CH4) and provide high temporal resolution. In our lab we use both approaches: IRMS for high‑precision δ13C and δ15N of gases and solids, and laser spectrometers for continuous δ13C‑CO2 and δ13C‑CH4 monitoring.(example).

Elemental Analysis
Elemental analysis quantifies total C, N and other elements in soils and plant tissues. Finely ground samples are combusted at >1,000 °C in O2. Helium carrier gas transports the products through a reduction tube where O2 is bound, NOx reduced to N2 and halogens trapped on Ag wool. The resulting gases (CO2, N2, H2O, SO2) are separated and detected, enabling qualitative and quantitative determination of C, N, H and S. We use a Flash EA to prepare samples for IRMS and to measure total C and N.

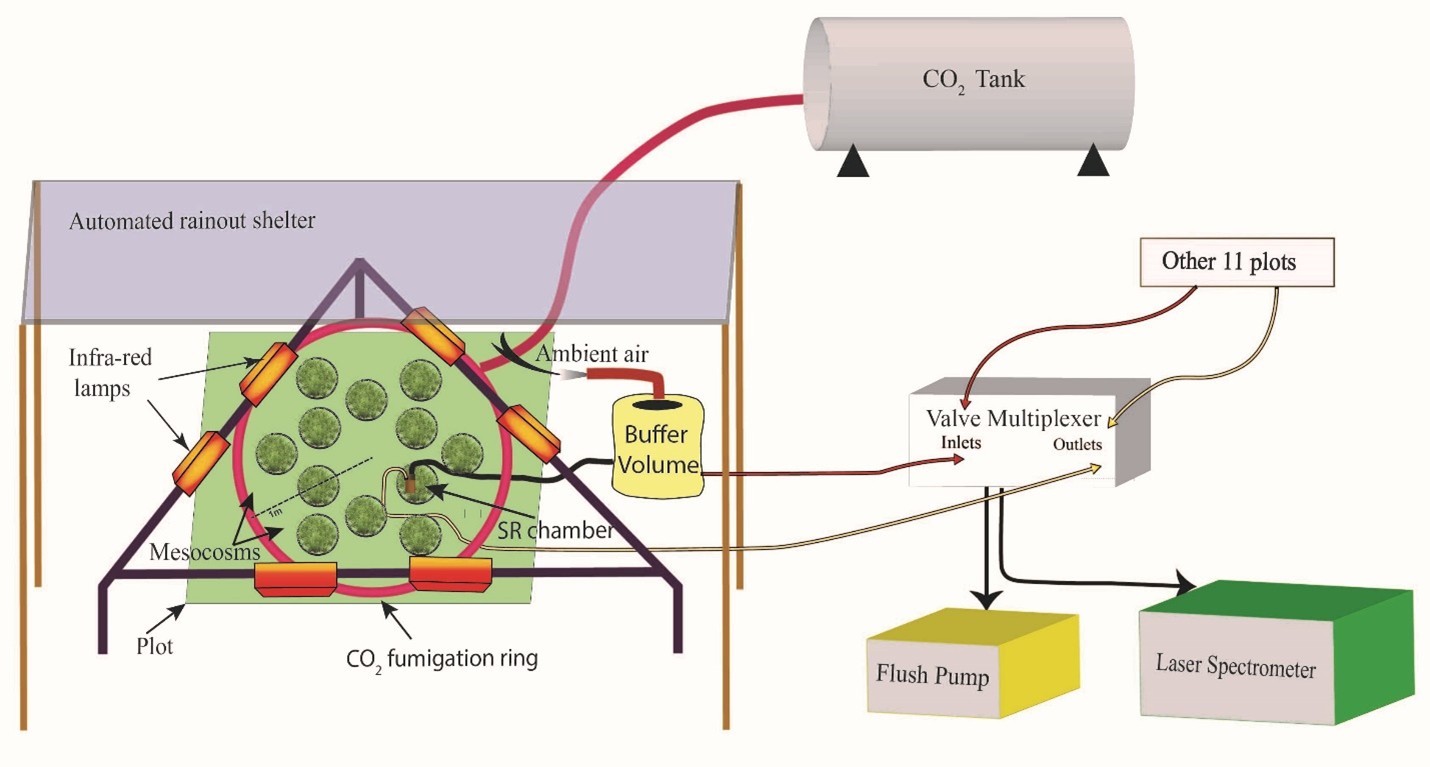
Gas Flux Measurements
We quantify soil and plant greenhouse‑gas fluxes using manual chambers and automated multiplexed systems. Automated chambers provide high‑frequency time series with precise timing; manual systems are versatile and portable for campaigns.